
India stands with its friends, says Jaishankar as Indian-made vaccines arrive in Afghanistan
The COVID-19 pandemic triggered a global health emergency and severely impacted both the public and private health sectors. On January 30, 2020, India reported its first case while The World Health Organization (WHO) proclaimed it a global pandemic and a public health emergency of international concern on March 11, 2020.
For fighting this global pandemic, the government announced a nationwide lockdown for 21 days as a preventive measure. But with time situation was getting worse & cases were at the peak, due to which lockdown was gradually increased. Fear of a pandemic, as well as attempts to prepare for fighting the pandemic, it prompted adjustments in our operating procedures.
Vaccine invention and inoculation drive
As soon as the epidemic began, doctors and scientists began studying and researching the virus in order to produce the most effective treatment techniques and drugs. There were immense debates regarding the best vaccine that would immunize our bodies to fight against this deadly virus. With their mindful approach, experts & scientists created Covishield & Covaxin vaccine.
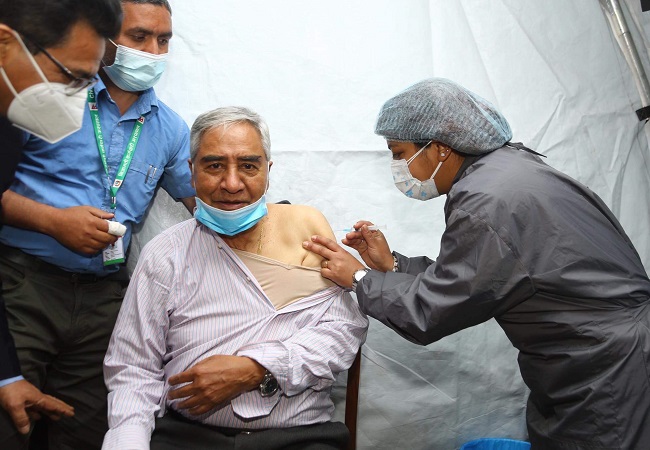
At All India Institutes of Medical Sciences (AIIMS) in Delhi, India’s first COVID-19 vaccine candidates began a human trial. The initial phase of Covaxin’s human clinical trial enrolled almost 2,000 people. After the successful trial on January 16, India began administering vaccines to frontline employees and those over the age of 60.
Due to a lack of education in India, many people opposed the vaccination drive, stating that it would kill them and that getting vaccinated would put them at greater risk. There were a variety of challenges ranging from planning to infrastructure to misinformation.
Import & Export of Vaccines
Due to sudden demand for vaccines, India had to take help from other countries in order to fulfill the supply for all the citizens to get vaccinated. Sooner, the country’s drug agency cleared the route for the import of Covid-19 vaccinations by issuing a set of instructions. Sputnik V, a Russia-made vaccine, was the first imported vaccine to be accessible in the private market.
The emergency use of imported vaccines to curb the spread of coronavirus infections was allowed by the government. Manufacturers had to deliver 50 per cent of their monthly doses to the government and the remainder to state governments or the open market under the third phase of its immunization policy.

Not only government but also the business community in India has been instrumental in promoting COVID-19 vaccination by assisting in the provision of vaccines to employees and their families at no cost or at subsidized rates.
Not just India sought help, but way before that since January, India has delivered 66 million vaccines overseas, enough to vaccinate the whole cities of Delhi, Mumbai, and Kolkata. Approximately 2 million doses were shipped out of the country in April alone. On April 22, the most recent shipment arrived in Paraguay, the same day that India set a new world record for new infections.
The government has been cautious in opening of workplaces and marketplaces. The vaccination process has been expedited, which will aid citizens in their fight against the new variants “Delta” or “Delta Plus.”
COVID instructions, which include wearing a mask, keeping safe distance, maintaining excellent hygiene, avoiding unnecessary meetings, and staying at home, remain unchanged in wake of the spread of contagious virus.




