
New Delhi: India is soon going to have its domestic production of anti-viral Remdesivir drug, which would have safety, efficacy and stability for “restricted emergency use” on COVID-19 patients.
The Drug Controller General of India (DCGI) has recently given approval to Remdesivir for “restricted emergency use” on severely ill hospitalised coronavirus patients.

“Remdesivir is a drug that is still under trial. Hence, looking at the COVID-19 situation in the country, it has been permitted for ‘restricted emergency use’ on COVID-19 patients. Doctors have to get the consent-form duly filled by the patient in a prescribed manner before using it,” senior govt official stated, adding that it is the first drug in India which has such strong control.
“The drug will be administered in the form of infection in five doses. On the first day, a patient will be given two doses followed by one dose for the next four days under the strict monitoring of doctors. The drug has to be given only to those severe COVID-19 patients with oxygen saturation level below 94 and respiratory rate more than 24,” the senior government official further said.
He informed that in other countries, patients are being administrated with 10 doses of Remdesivir.
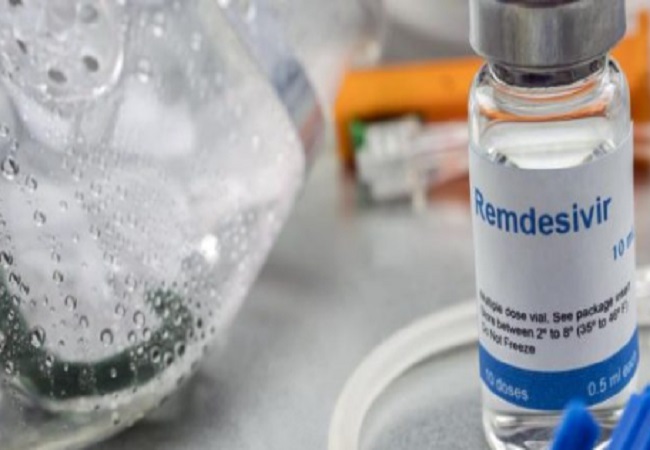
Remdesivir, an investigational antiviral therapy developed by Gilead, received Emergency Use Authorisation (EUA) by the US Food and Drug Administration (USFDA) to treat COVID-19. However, multiple additional clinical trials are ongoing to generate more data on the safety and efficacy of the drug as a treatment for the virus.
Remdesivir helps to decrease hospital stay
Sharing insight on the Remdesivir drug, the AIIMS Director Dr Randeep Guleria said that the drug helps to decrease hospital stay but the benefits don’t reflect as far as death or mortality benefit is concerned.

“There is no definite anti-viral drug which has been proved as far as COVID-19 is concerned. A lot of research is going on. There are some anti-viral drugs which are being used, one of them is ‘Remdesivir’ that is being made by a company from the US. Data suggests that it helps to decrease hospital stay but it doesn’t show benefit as far as death or mortality benefit is concerned. So we need more data to suggest that these drugs are useful or not,” said Dr Guleria in an interview to ANI. “Remdesivir is used as per the guidelines of emergency use authorisation. There is also very limited stock available. I am hopeful that in coming weeks we have larger doses of it,” he added.
Dr Guleria explained about the ways in which COVID-19 patients are being treated. He said that hydroxychloroquine (HCQ) is the commonly used drugs to treat the patients.
“Repurposed drugs are available in the market and studies, too, have suggested that they may have anti-viral activities and therefore they can be used. The drug which we use commonly for treating coronavirus patients is hydroxychloroquine (HCQ). There are controversies regarding the drug but data suggests that it may be useful especially in the early days,” he said.
(With ANI inputs)




